Explain How Isotopes of an Element Are Similar and Different
According to the modern periodic law the physical and chemical properties of elements are the periodic functions of their atomic numbers. Answered 5 years ago.

Difference Between Compound And Mixture Definition Characteristics Types Of Bonding Compounds And Mixtures Study Chemistry Chemistry Classroom
An isotope of an element is different from.
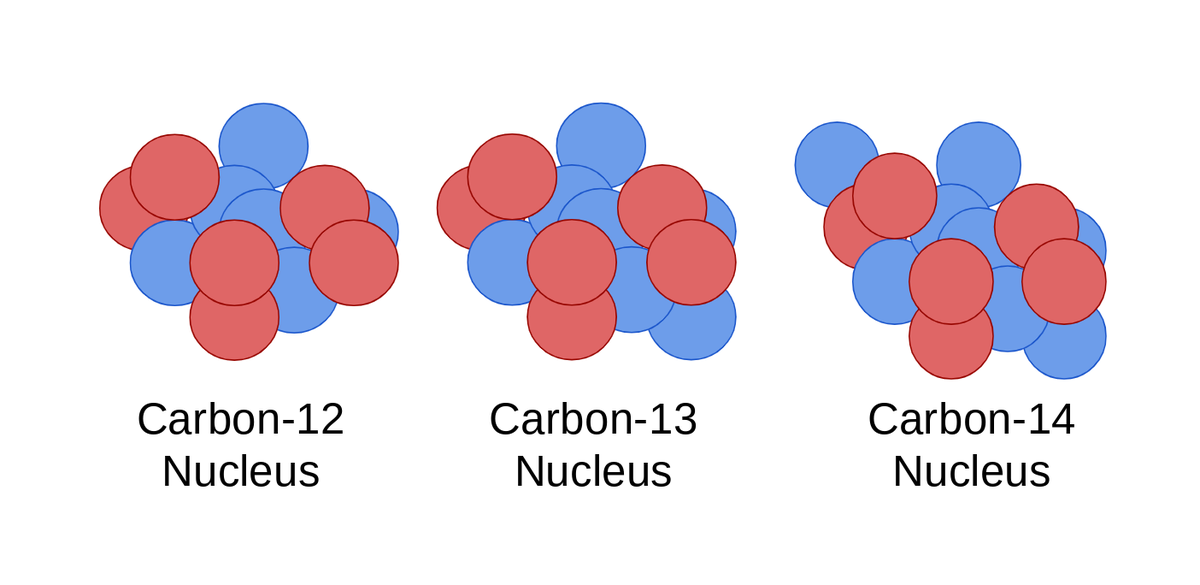
. Chemical reactions involve changes to the electrons in atoms but not changes to its protons or neutrons. Isotopes of the same element must have same number of protons but different number of neutrons and hence they have different mass. Different isotopes have different numbers of neutrons in their nuclei resulting in different atomic weights for the different isotopes of a single element.
Example- 612C and 613C atomic number View the full answer. All the Isotopes of an Element Have Identical Chemical Properties. The exceptions are the isotopes of hydrogen since the number of neutrons has such a significant effect on the size of the hydrogen nucleus.
Hence isotopes have similar chemical properties but different physical properties. The chemical properties of isotopes of a single element tend to be nearly identical. Mass number determines the physical properties.
Explain how two isotopes of an element are similar. However they have different numbers of neutrons which affects the mass number. 12-carbon will have 6 protons and 6 neutrons while 14-carbon will have 6 protons and 8 neutrons.
An isotope of an element is same as the element in that it has the same number of protons. All atoms of that element will have the same number of protons but neutrons can vary. Since the neutron number is different their mass number also differs.
Isotopes are elements with different masses. Isotopes of the same element have the same number of protons and electrons when in neutral atomic form. Every element on the periodic table has multiple isotope forms.
Isotopes are the atoms of an element with the same atomic number but different mass number. Isotope behavior only differs when a reaction involves neutrons. The physical properties of any isotope are largely determined by its mass.
Two different isotopes of the same element have the same number of protons but each has a different number of neutrons in its nucleus. The difference between C12 and C13 is the amount of neutrons both have 6 protons where C12 have 6 neutrons whereas C13. Isotopes of an element possess the similar number of protons and hence same number of protons and have the same number of electrons since the electrons are responsible for chemical properties isotopes of an element show similar chemical properties.
Also the isotopes of same element are not equally abundant in nature. Thats what an isotope is. Isotopes of the same element differ only in neutron numbers.
When it comes to physical properties of isotopes including mass melting or boiling point density and freezing point they are all different. This is because isotopes of an element have the same number of electrons as an atom of that elementBut they have different number of neutrons which affects the mass number. Explain how all of the isotopes of an element are similar and how they are different.
As an example carbon has the elemental number 6 which means it has 6 protons. 1 See answer Advertisement Advertisement yummcookies is waiting for your help. Nuclear reactions involve changes to the protons or neutrons in an atoms nucleus but not changes to its electrons.
However the isotopes of the same element have the same number of protons. These different versions of the same element are called isotopes. The different number of neutrons affects the mass number.
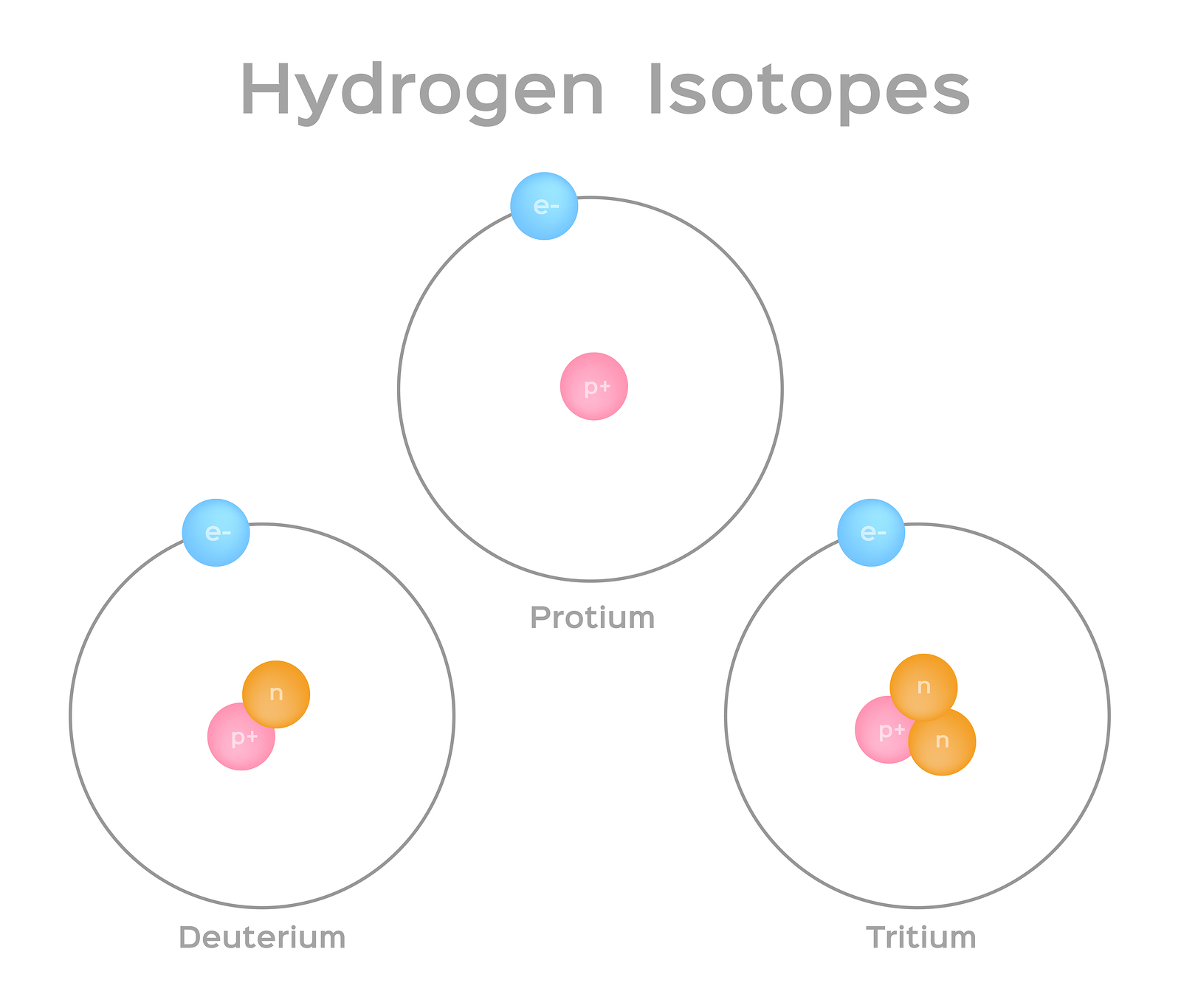
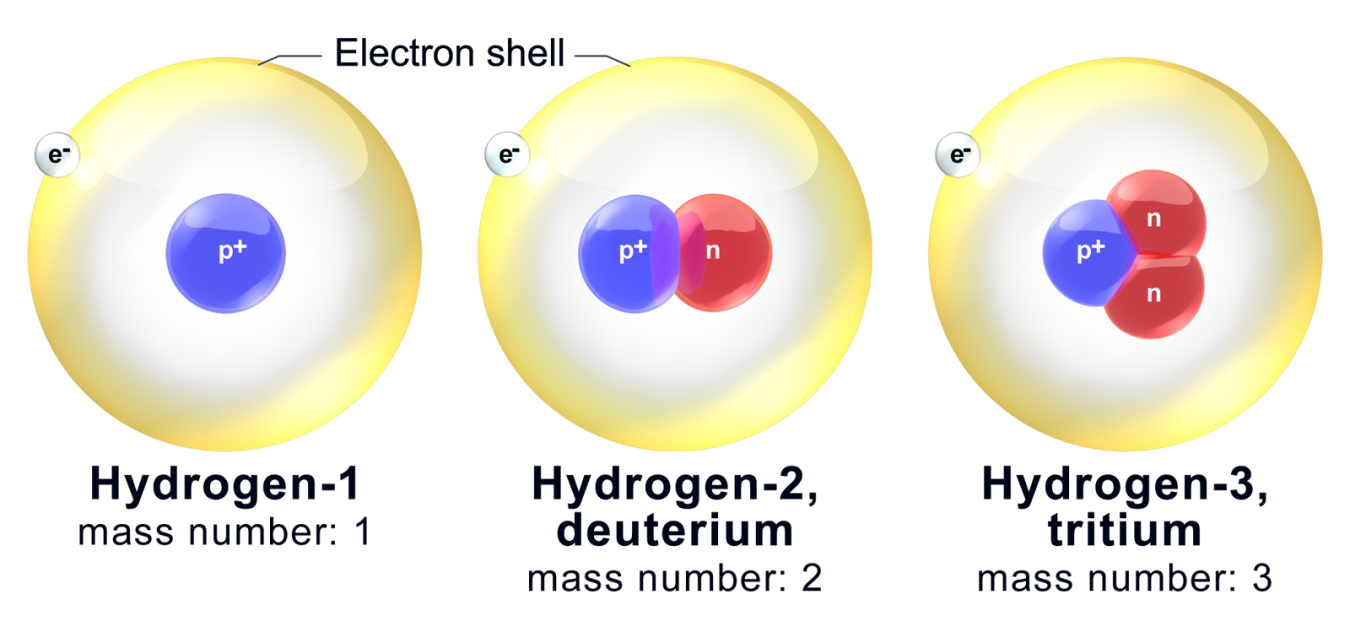
All three isotopes of hydrogen have identical chemical properties. Yummcookies yummcookies 10122021 Biology High School answered Please answer asap. Lets use flourine again.
In a given element the number of neutrons can be different from each other while the number of protons is not. Thus isotopes having the same atomic numbers have similar chemical properties. They have similar chemical properties because isotopes of an element have the same number of electrons as an atom of that element.
Isotopes of an element have different physical properties because they have different mass numbers. Chemical properties of different isotopes are almost similar. All the Isotopes of an element have identical chemical properties because they have the same number of electrons as an atom of that element but they have different numbers of neutrons.
These different atoms of the same element are isotopes. A ions are formed by their atomic number but their mass is es are the atoms of the same element with the same atomic number but different mass numbersThe number of electrons protons and neutrons of the isotopes differs based on their ionization systemDeuterium protium and tritium for example. Different isotopes of the same element are chemically the.
The electron arrangement is the same owing to same chemical properties. What Are Isotopes Explain By Giving An Example. Explain how all of the isotopes of an element are similar and how they are different.
Same element different amounts of neutrons. All atoms of a given element have the same number of protons but some atoms have more neutrons than other atoms of the same element and therefore have a. Mass number determines the physical properties such as.
This is because the number of electrons determines chemical properties and all three isotopes have one electron in their atoms. An isotope is a chemical structure that is highly similar to its parent isotope with the difference lying in the number of neutrons that the compound has. Atoms of the same element can be different from each other.
Isotopes of an element are those which have similar atomic number but different mass number. Let us consider an example of isotopes for hydrogen there are three type of isotopes which are protium 1 H 1. They are different from each other by having a different number of neutrons.
Carbon have two stable isotopes found in nature one is C12 and one is C13 C14 does also exist by not stable form of carbon therefore not found in nature - produced under nuclear reaction. Explain how they are different.

Isotopes And Atomic Mass Activity Eggium Mass Activities Petri Dishes Activities

How To Calculate Relative Abundance Abundance Calculator

What Is An Isotope Examples Types How To Identify An Isotope Video Lesson Transcript Study Com
Isotope Basics Nidc National Isotope Development Center

Isotope Basics Nidc National Isotope Development Center

Pin By Mills On My Notes In 2022 Mass Number Protons Writing

These Are Three Isotopes Of Lithium They Are Different Because The Mass Number Is One Mo Teaching Chemistry Physical And Chemical Properties Science Chemistry

A Frayer Model Is A Perfect Tool To Deepen The Understanding Of Isotopes Students Will Define Isotopes Show I Human Body Projects Teacher Guides Atom Diagram
Doe Explains Isotopes Department Of Energy

Difference Between Isotopes And Elements Compare The Difference Between Similar Terms

What Is An Isotope In 2022 Secondary School Science Secondary Science Teacher Notes

What S The Difference Between Chemical Reactions And Nuclear Reactions And What Does That Mean For Nuclear Reaction Chemical Reactions Graphic Design Lessons

Isotopes Radiation Radiation Nuclear Radiation Element Symbols

Isotopes And Atomic Mass Teaching Chemistry Secondary Science Classroom Chemistry

Labeled Parts Of An Atom Diagram Atom Diagram Atom Worksheets

Isotopes Are Variants Of A Particular Chemical Element Which Differ In Neutron Number And Consequently In Nucleon Online Lectures Online Study Study Materials

Difference Between Isotopes And Elements Compare The Difference Between Similar Terms


Comments
Post a Comment